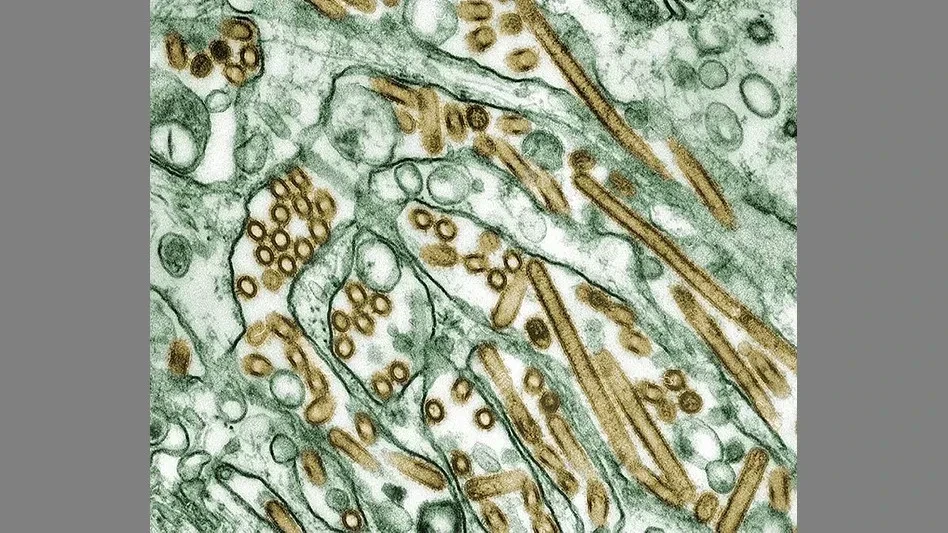
Photo courtesy CDC
Last December, the U.S. Food and Drug Administration (FDA) initiated an assignment sampling 60-day aged raw milk cheese to determine if it is effective at reducing or eliminating viable H5N1, also known as highly pathogenic avian influenza or bird flu.
The first sample was taken Jan. 2, and sample collections are anticipated to be complete by the end of March. As of March 10, 110 samples of the planned 299 have been collected. Of those 110 samples, 96 were negative by PCR (meaning that H5N1 was not detected in the analyzed samples), and 14 are still in progress. Final results are expected later this spring.
Raw milk cheese is made with unpasteurized milk. In the U.S., cheese allowed to be made from raw milk must be aged for a minimum of 60 days to mitigate the risk from any pathogens, if present.
Latest from Quality Assurance & Food Safety
- Kim Heiman Elected to Second Term as President of Wisconsin Cheese Makers Association
- FAO Launches $150 Million Plan to Restore Ukrainian Agricultural Production
- Pet Food Company Implements Weavix Radio System for Manufacturing Communication
- Penn State Offers Short Course on Food Safety and Sanitation for Manufacturers
- USDA Announces New Presidential Appointments
- FDA to Phase Out Petroleum-Based Synthetic Dyes in Food
- IFT DC Section to Host Food Policy Event Featuring FDA, USDA Leaders
- CSQ Invites Public Comments on Improved Cannabis Safety, Quality Standards





