Editor's Note: To vote in our new reader poll about industry event attendance, head here.
In January, FDA released its proposed redesign of Human Foods Program, which is a response to calls from many to improve the way the agency handles food safety.
"Today I am announcing a new, transformative vision for the FDA Human Foods Program," Robert M. Califf, M.D., MACC, Commissioner of Food and Drugs, said at the time. "I am also announcing a transformative vision for the Office of Regulatory Affairs (ORA, the FDA’s field-based operations) to support the FDA organization as a whole. The proposed structures for both groups will have clear priorities that are focused on protecting and promoting a safe, nutritious U.S. food supply that more quickly adapts to an ever-changing and evolving environment. I believe this proposed approach addresses the recommendations outlined in both reports, and takes into consideration feedback from stakeholders, as well as the voices of employees working in the Human Foods Program who had an opportunity to share input through numerous interactive and listening sessions over the past month. Creating a Human Foods Program under a single leader who reports directly to the Commissioner unifies and elevates the program while removing redundancies, enabling the agency to oversee human food in a more effective and efficient way."
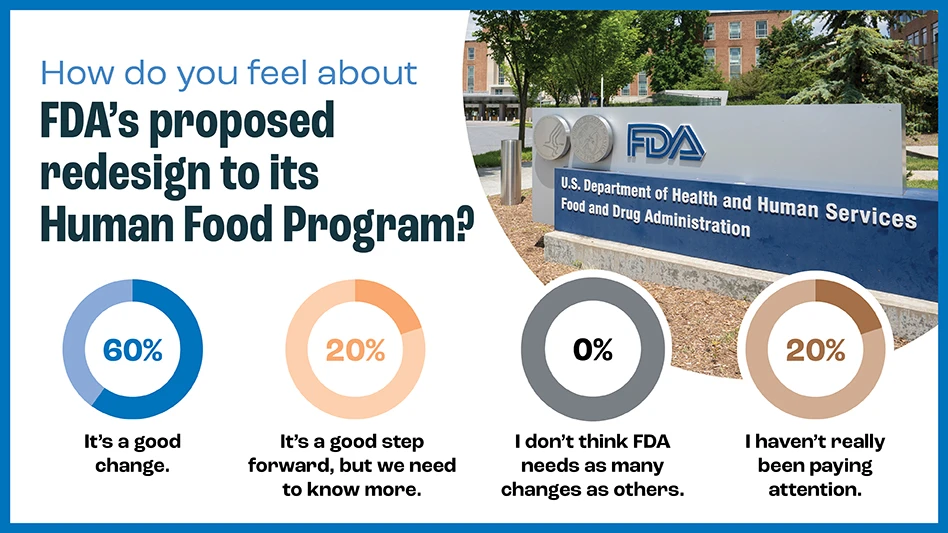
In a recent poll, a majority (60%) of QA readers think the changes are good, while 20% need to know more about the plans.
In a statement after the proposed redesign was released, Steven Mandernach, executive director, Association of Food and Drug Officials. echoed both sentiments.
"We see many positive aspects to the proposal released today by FDA Commissioner Robert M. Califf, M.D., outlining a new vision for the FDA Human Foods Program and the Office of Regulatory Affairs to support the FDA organization as a whole," Mandernach said at the time.
"In particular, we appreciate that his proposal elevates state and local programs who do much of the food safety work, creates a deputy commissioner with streamlined authority, and focuses the agency’s nutrition mission in a Center of Excellence for Nutrition.
"We see additional opportunities to streamline the authority between the Deputy Commissioner for Human Foods and the Office of Regulatory Affairs and will continue to engage with the Commissioner on this issue."
More recently, foodborne illness attorney Bill Marler has started a campaign advocating larger changes. Marler has launched the "Get the F Out of the FDA" advertising campaign to urge Congress and the White House to break apart the FDA and create a new dedicated foods agency focused on both food safety and nutrition.
"It is time the White House and the Congress take responsibility for protecting the public by breaking the FDA apart and creating a new dedicated foods agency," he said in a release. "However, while passing this legislation, the White House and the Congress should immediately direct the Commissioner to unify all parts of the foods program and budget under an empowered and accountable Deputy Commissioner."
Whether it's a new agency or major reforms to the FDA program, it seems clear that most people agree changes need to be made.
Latest from Quality Assurance & Food Safety
- FDA Foods Coalition Urges RFK Not to Cut More Resources, Staff
- Understanding Rodents and Bird Flu
- Bird Flu: What FSQA Professionals Need to Know
- Registration Open for 129th AFDO Annual Educational Conference
- Frank Yiannas, Aquatiq Partner to Expand Global Reach of Food Safety Culture
- World Food Safety Day 2025 Theme: Science in Action
- Ancera Launches Poultry Analytics System
- USDA Terminates Two Longstanding Food Safety Advisory Committees





