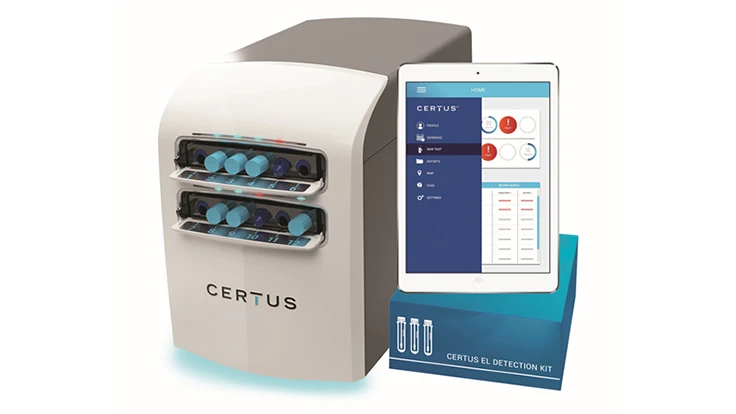
Certus, an innovator in food safety technologies, has signed an exclusive license agreement with the global medical technology company, Becton, Dickinson and Company (BD), for use of its rapid pathogen-detection technology. Enabled by BD's proprietary SERS nanoparticle technology, the newly introduced Certus System will make food safer by providing small to mid-sized producers fast, easy and cost-effective ways to detect environmental Listeria in food processing environments and the food itself prior to reaching consumers, the company said.
"This is a critical agreement for Certus as BD's technology provides the foundation of our patented Grow, Read, Detect system giving producers real-time monitoring through simultaneous enrichment and detection," said Certus President John Coomes. "We believe our approach, backed by BD's long-standing expertise in nanotechnology, is a significant industry breakthrough that radically simplifies what was once a complex process. This will enable any food processor to conduct safe and easy on-site testing, receive instant alerts, and take action to remediate any issues."
BD's exclusive license to Certus also includes technology to enable pathogen detection in a bio-contained tube during enrichment so that the facility where the testing is conducted is not exposed to enriched pathogens. BD continues to work with partners to further develop the SERS technology and is the supplier of SERS nanoparticles used in Certus products. The first commercial Certus System will be launched in 2018 for environmental monitoring and prevention of Listeria spp.
"The beauty of our system is that anyone with minimal training will simply swab a surface, add media to our Bio-Lock Detection Tube, and insert the tube into the detection unit to start getting rapid results," Coomes said. "There is no need for centrifuges, incubators, pipettes, stomachers, bags or other ancillary items and steps that current methods require."
Since its IAFP debut earlier this year, Certus has developed multiple prototypes which are now being used in the U.S. and Europe for optimization of the Certus EL Detection kit. Further development of the system will involve seeking AOAC validation and launching a research and development program for Certus Environmental Salmonella Detection Kits. For more information on Certus visit certusfoodsafety.com.
Latest from Quality Assurance & Food Safety
- Chef Robotics Introduces Pat-Down Capability for Meal Presentation and Sealing
- USDA Launches Regenerative Pilot Program
- Indoor Ag-Con Adds Food Safety Track to Conference Lineup
- IDFA Recognizes Federal Officials for Support of U.S. Dairy Industry
- Tetra Pak Acquires Bioreactors.net
- Fresh Del Monte Receives Rabobank Leadership Award
- São Paulo Earns Guinness World Record for Largest Municipal Food Security Program
- KPM Analytics Releases Ready-to-Use NIR Calibration Packages





